Amino Acids, Proteins, and DNA Notes
This unit can be broken down in to the following;
· Amino Acids
· Proteins
· DNA
· Enzymes
· Cisplatin
Amino Acids
There is many amino acids but 20 present in the human body, some of these are presented on data sheets and are the ones most likely to be asked about.
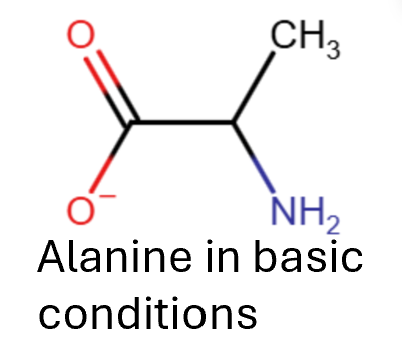
An amino acid consists of a carboxylic acid and an amine functional group so it can act as both a base, proton acceptor, and an acid, proton donor. Alanine is a simple amino acid, if it is acidic solutions so will accept a proton, acting as a base, and we get the following.
In alkaline conditions it will behave as an acid and donate a proton giving the following. If there is any other functional groups which are acidic they will also be affected.
In neutral conditions the carboxylic acid can lose its proton and the amine can gain a proton producing two charges at the same time, a zwitterion. Zwei being German for two. This means that amino acids behave in the same way as ionic substances, high melting points and very soluble.
Proteins
Amino acids can combine to form amides, when discussing proteins this can be referred to as a peptide. Two amino acids joined together are referred to as dipeptide, three are tripeptides which are both within the scope of the course. Many amino acids combined may also be referred to as polypeptides.
Proteins are made from polypeptides, the primary structure of the protein is the order of amino acids in the polypeptide. For example the polypeptide above has the primary structure of ala-ala-ala.
The secondary structure of proteins is either an alpha-helix or beta-pleated sheet. These shapes are held together with hydrogen bonds between the amino acids, between the lone pairs of oxygen in the carbonyl and the hydrogen on the nitrogen, this interaction is also in the tertiary structure indicated below.
The tertiary structure of proteins is the folded alpha helix and beta pleated sheets. The tertiary structure is held together by
Hydrogen Bonds Between C=O and N-H
Ionic Bonds between RCOO- and RNH3+
Disulfide bridges
Disulfide bridges are produced when two sulfur containing amino acids, cysteine, react and form a R-S-S-R functional group.
Hydrolysis of Polypeptides
Hydrolysis, addition of water, to a peptide linkage will convert the molecules back to a carboxylic acid and an amine and ultimately back in to amino acids.
The conditions required for hydrolysis of a peptide are boiled with 6M hydrochloric acid.
Chromatography of amino acids
Once a peptide has been completely hydrolysed the constituent amino acids can be identified by thin layer chromatography. Amino acids will produce colourless spots, they can be identified by UV light or stained with Ninhydrin to make them observable. The Rf value is then used to identify the amino acids.
Enzymes
Enzymes are large proteins which are biological catalysts, each enzyme is structured for a different reaction. An enzyme will have a part of the protein which is referred to as the active site, this is the part of the protein which is unique to each reaction this model of enzyme action is often called lock and key.
The reactants will bind with the active site, this will reduce the activation energy often by changing the shape slightly of the reactants.
With the activation energy lowered the reaction can now take place, the products are then released from the enzyme which can now catalyse another reaction.
As the active site is specific to the shape of a molecule it is also stereoselective, it may react with one molecule but not an enantiomer of it.
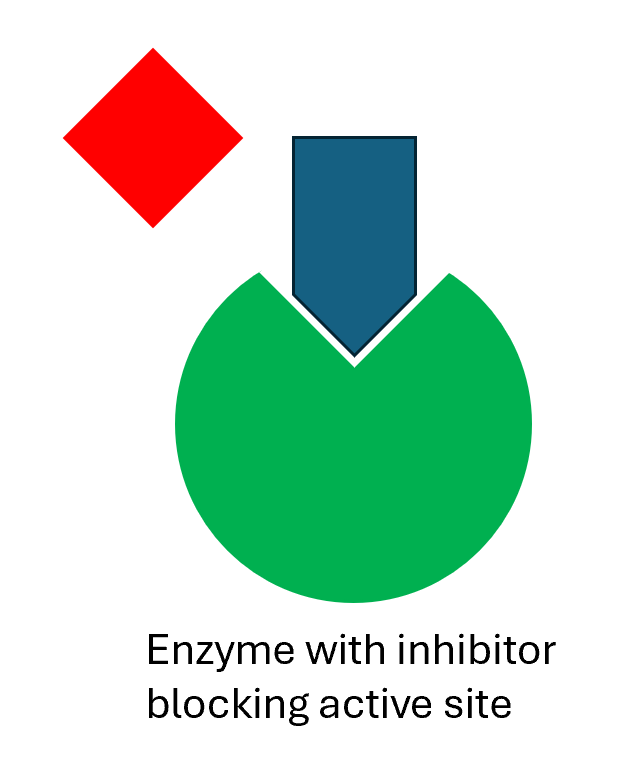
Enzyme Inhibitors
Drugs can be used to inhibit, stop, the enzyme working they can be permanent or temporary. Temporary inhibitors will sit in the active site of an enzyme so no reactants can enter it. The active site can also have its shape permanently changed by an enzyme inhibitor so it can not accept the reactants.
Drugs used for enzyme inhibitors are often designed and modelled by computers on how they interact with the enzyme before being produced and tested.
DNA
DNA is a polymer made from the monomer nucleotides, a nucleotide has three parts.
Phosphate Ion
2-deoxyribose
Base (Cytosine, Thymine, Adenine, or Guanine)
The three parts are bonded together, the location bonded in the bases is highlighted above, to produce the following;
The nucleotides are then polymerised by condensation reaction to form DNA.
The base pairs either have 2 or 3 hydrogen bonds between each other, this means that the chain of DNA can have a complimentary chain, this will aid replication.
Cisplatin
Cisplatin is an anti-cancer drug, it bonds with 2 guanine bases and will stop replication of DNA so destroys the cell. A cancerous cell will replicate more rapidly than most healthy cells so is more affected by the cisplatin and will reduce in size.