Aromatic Chemistry Notes
Aromatics can be broken down in to the following sections;
· Structure of Benzene
· Evidence of Structure
· Electrophilic Substitution
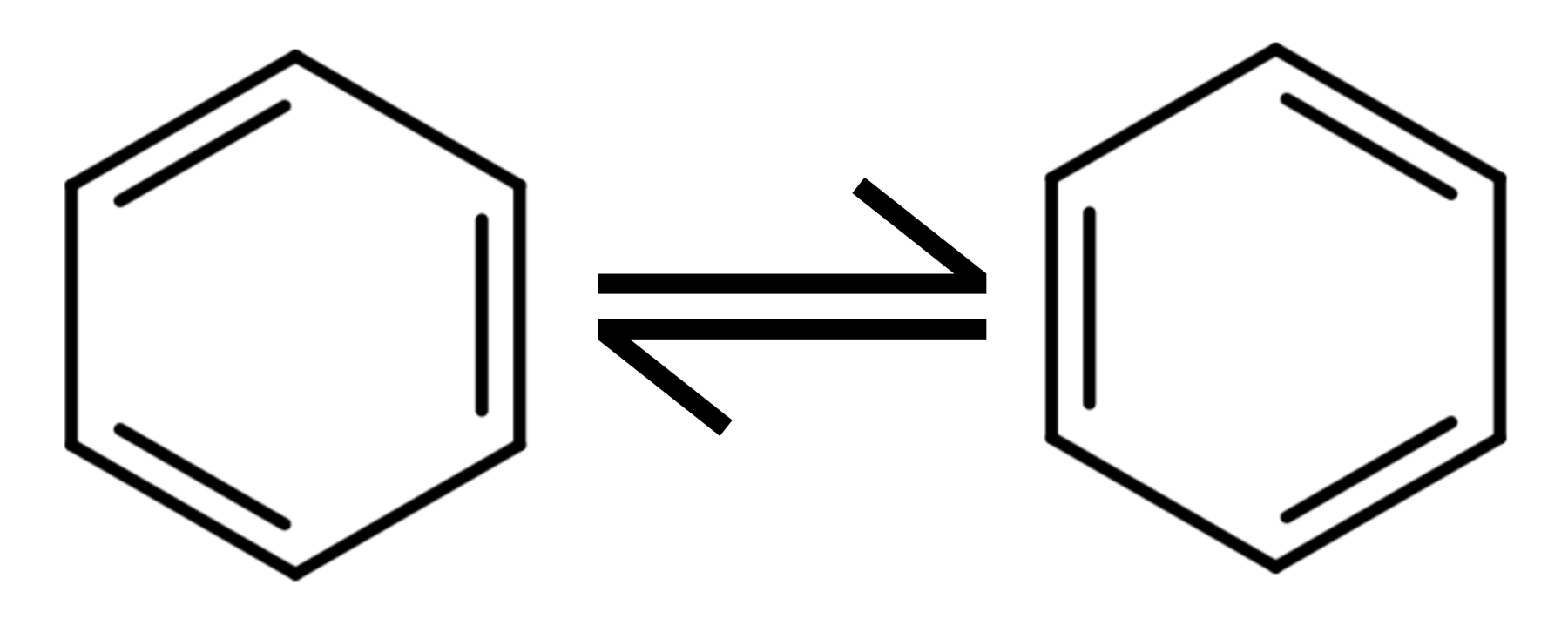
Benzene
Benzene, C6H6, is a commonly studied aromatic hydrocarbon, an arene. The structure is of a six-member ring and often drawn with alternating single and double bonds, even though not correct. Kekule’s model of benzene would have the IUPAC name of cyclohexa-1,3,5-triene.
The delocalised model of benzene is used due to the reasoning below. Benzene is a planar molecule with the shape around each carbon being trigonal planar and a bond angle of 120. To build the current model of benzene we first start with a six-member ring of carbons and between each of the carbons there is a single sigma bond. This sigma bond is produced by overlapping p orbitals and is equivalent to most single covalent bonds.
In ethene there is p orbitals which go above and below the plane these orbitals overlap which produce a pi bond, the second bond in a double bond. A very similar process in benzene occurs. On each carbon within the benzene ring there is a filled p orbital above and below the plane these will overlap producing a ring of pi bonds, the electrons become delocalised in this ring of overlapping p orbitals.
The overlapping of the orbitals and then delocalisation of the electrons makes benzene a more stable molecule than expected. Any molecule with alternating single and double bonds between carbons becomes more stable, this is known as conjugation.
Evidence of structure
Kekule’s structure of benzene has two significant pieces of evidence which contradict the alternating double and single bond structure. The first being bond length, the bond lengths of C-C bonds are given below.
The carbon-carbon single bond length is longer than the carbon-carbon double bond length, this would lead to Kekule’s model being an irregular hexagon.
When the bond length of a carbon-carbon bond in benzene has been measured it gives the length of 140 pm, which is the same to neither the single or double bonds in carbon-carbon. The shape of benzene when studied is also a regular hexagon indicating all the bond lengths are the same. This evidence indicates that Kekule’s model is incorrect and he bond are not alternating but are actually all equal. Kekule’s model was improved by stating there is resonance and the double bonds are able to move and create resonance hybrids, a mixture of two version.
Evidence for delocalised electrons
If we are to hydrogenate the below molecules we may expect the same energy change.
In reality the hydrogenation of hex-1,4-diene is -240kj/mol which is which we may have expected know the previous data, the hydrogenation od hex-1,3-diene is -232kj/mol which is more than expected. This difference in energy indicates that the hex-1,3-diene is more stable than the hex-1,4-diene and this is due to the electrons being delocalised between the alternating single and double bonds.
A significant piece of information which indicates the delocalised model being correct is the thermal stability of benzene. The enthalpy of hydrogenation, adding hydrogen across a double bond, for cyclohexene is -120kj/mol, in the molecule hex-1,3,5-triene there would be 3 hydrogens adding across 3 double bonds and the energy change would be 3x that of the previous reaction, -360kj/mol. When done experimentally the hydrogenation of benzene to cyclohexane is -208kj/mol. This difference indicates that benzene is more stable that hex-1,3,5-triene a further piece of evidence for the delocalised model.
Reactions of benzene
Benzene due to its increased stability of delocalised electrons has differing reactions that alkenes, for example it will not discolour bromine water or undergo electrophilic addition.
Benzene will react with electrophiles but will undergo electrophilic substitution to maintain the delocalised system to maintain stability.
Nitration of benzene
Benzene will undergo electrophilic substitution when reacting with a nitronium ion, the nitronium ion is produced by reacting concentrated nitric acid with concentrated sulfuric acid.
The sulfuric acid donates a proton to the nitric acid, which acts as a base, and the protonated nitric acid decomposes into water and a nitronium ion.
The benzene will then react with the nitronium ion, the electrons from the delocalised ring will react with the nucleophile
A transition state will then occur with a partially complete delocalised ring with a positive charge will be present. On the carbon which is now bonded to the nitrogen the bond to the hydrogen will break and the electrons go in to the delocalised ring. This will remake the delocalised ring and release a proton. The proton will combine with the hydrogen sulfate ion to regenerate the sulfuric acid catalyst.
This nitrobenzene can then be reacted with tin and hydrochloric acid to produce phenylamine.
Friedel-Crafts Acylation of Benzene
Ethanoyl chloride if turned into an electrophile can react with benzene in an electrophilic substitution. The electrophile is generated by ethanoyl chloride reacting with aluminium chloride to produce the acylium ion and aluminium tetrachloride.
Similar to the nitration above the electron in the delocalised ring will form a bond to the electrophile and form a positive charge in the centre of an incomplete delocalised ring.
The hydrogen on the reacted carbon will then leave as a proton which will reform the ring of delocalised electrons.
After the reaction has taken place the proton released by the benzene ring reacts with the aluminium tetrachloride to regenerate the catalyst.