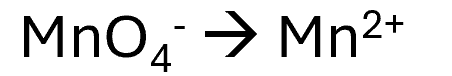Redox notes
Redox, Reduction Oxidation reactions, is split in to the two sections
· Oxidation States
· Redox Reactions
Oxidation states
Oxidation state or number is the number of electrons lost or gained by an individual atom within a compound compared to its element. The oxidation state of any element is zero, there are some we need to remember and use them to work out other oxidation states.
For example we could calculate the oxidation state of nitrogen in Nitric Acid, HNO3.
Hydrogen has a charge of +1, the 3 oxygens each have -2, the molecule is neutral overall so the oxidation states must add up to zero. The oxidation state of nitrogen must be +5.
Another worked example of Cr in K2Cr2O7
Redox Reactions
A species which gains electrons is reduced, it can also be regarded as an oxidising agent Below is the oxidation of Iron (II) to Iron (III)
A species which loses electrons is oxidised, it can also be regarded as a reducing agent. Below is the reduction of Iron (III) to Iron (II)
Producing Half equations
To produce half equations of single element equations in relatively simple, the number of atoms/ions must be balanced and then the charges by adding electrons for example chloride ions with iron (III)
When the oxidizing/reducing agent contains oxygen it makes balancing the half equation much more complex, but it can be done methodically.
1) Balance the species which is oxidised/reduced
2) Balance the oxygens
3) Balance the protons
4) Balance the electrons by identifying the change oxidation states
In this equation no balancing of Mn is needed.
There is 4 oxygen atoms on the left, so on the right we need to add 4 waters.
There is now 8 hydrogens on the right so there must be 8 protons added to the right hand side.
The oxidation states of the oxidised/reduced species is calculated so in the case +7 on the LHS and +2 on the RHS, we will need to balance this charge by adding 5 electrons to the LHS.
The half equation for dichromate can be produced in the same way.
Combining half equations
A classic reaction is the reaction between chloride and potassium manganate to produce chlorine, we can combine the two half equations of the redox reaction to get an overall reaction. To combine two half equations you must first make the electrons equal.
The electrons will both need to be made to 10 as the lowest common multiple
The entire first half equation has been double so there is 10 electrons, the second equation multiplied by 5. they are now added together and the electrons cancelled down.